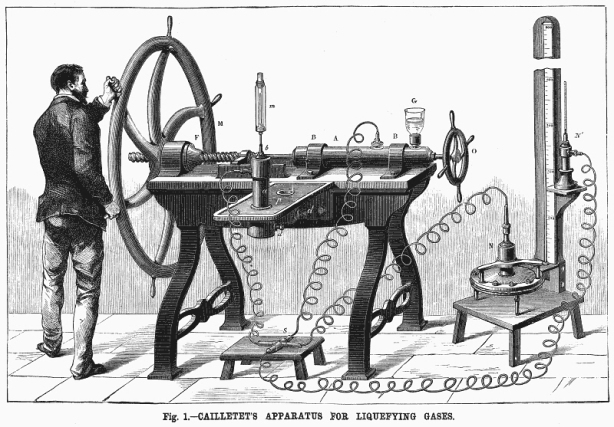
On Monday 3 December 1877, the French Academy of Sciences received a letter from Louis Cailletet, a 45 year-old physicist from Châtillon-sur-Seine. The letter stated that Cailletet had succeeded in liquefying both carbon monoxide and oxygen.
Liquefaction as such was nothing new to 19th century science, it should be said. The real news value of Cailletet’s announcement was that he had liquefied two gases previously considered ‘non condensable’.
While a number of gases such as chlorine, carbon dioxide, sulfur dioxide, hydrogen sulfide, ethylene and ammonia had been liquefied by the simultaneous application of pressure and cooling, the principal gases comprising air – nitrogen and oxygen – together with carbon monoxide, nitric oxide, hydrogen and helium, had stubbornly refused to liquefy, despite the use of pressures up to 3000 atmospheres. By the mid-1800s, the general opinion was that these gases could not be converted into liquids under any circumstances.
But in 1869, a paper appeared in a British journal which caused the scientific community to rethink its view.
The paper, entitled “On the Continuity of the Gaseous and Liquid States of Matter” and published in the Philosophical Transactions of the Royal Society, was written by 55-year-old Thomas Andrews, the vice-president of Queen’s College Belfast in Northern Ireland.
In addition to his administrative role, Thomas Andrews was also professor of chemistry at Queen’s College Belfast. From the start of his long professorial career he took an interest in gases, beginning with a study of ozone conducted jointly with the Scottish mathematical physicist Peter Guthrie Tait. Then in the summer of 1860, Professor Andrews turned his attention to the liquefaction of gases, a subject that the influential Michael Faraday had brought into the scientific spotlight during the 1820s; Faraday had been the first to liquefy chlorine gas in 1823.
Not surprisingly perhaps, Thomas Andrews went for the big prize in his initial experiments, in which he attempted to liquefy the ‘non condensable’ gases. And not surprisingly, he got absolutely nowhere – none of these gases showed any willingness to liquefy. Andrews then refocused his research on the liquefaction of carbon dioxide [called carbonic acid in his day], and in 1863 made the observation that would set him on the path to fame.
He wrote: “On partially liquefying carbonic acid by pressure alone, and gradually raising at the same time the temperature to 88° Fahr. [31.1°C], the surface of demarcation between the liquid and gas became fainter, lost its curvature, and at last disappeared. The space was then occupied by a homogeneous fluid, which exhibited, when the pressure was suddenly diminished or the temperature slightly lowered, a peculiar appearance of moving or flickering striæ [stripes] throughout its entire mass. At temperatures above 88° no apparent liquefaction of carbonic acid, or separation into two distinct forms of matter, could be effected, even when a pressure of 300 or 400 atmospheres was applied.”
Andrews had discovered the existence of a fundamental property of gases, which he called the “critical temperature” – the temperature above which no gas could be liquefied by pressure alone. If all gases had a critical temperature, then all gases could be liquefied if cooled below that temperature. The gases deemed ‘non condensable’ were simply gases whose critical temperatures were lower than the lowest achievable temperature at that time, which was around –110°C. What was needed was a new cooling principle to enable lower temperatures to be reached.
The good news was that the new cooling principle was already known to science. It had been discovered by James Joule and William Thomson (later Lord Kelvin) in a Manchester cellar a decade earlier.
– – – –

William Thomson, later Lord Kelvin (left), James Joule and their famous hand pump. The Joule-Thomson effect is named after them, as are the SI units of thermodynamic temperature (kelvin) and energy (joule).
In May 1852, James Joule and William Thomson conducted a famous experiment in the basement of Joule’s home in Salford, Manchester, England, in which they pumped pressurised air at a steady rate through a coil of lead pipe which was narrowly constricted at a certain point along its length and open to the atmosphere at its far end.
The apparatus was equipped with thermometers to measure the temperature of the airflow on either side of the constriction, which was insulated to prevent heat exchange with the surroundings.
Joule and Thomson observed a lowering of temperature. The air was cooled as it flowed through the narrowed section of the pipe, from a region of higher pressure to a region of lower pressure.
The discovery of this cooling effect, called the Joule-Thomson effect in their honour, was a landmark moment in the history of physical science and opened the way to cryotechnological applications of great scientific and commercial importance.
The hand pump which formed part of the original apparatus used by Joule and Thomson in 1852 is now in the collection of the Museum of Science and Industry in Manchester.
– – – –
Good fortune has smiled on many along the path of scientific discovery, and Thomas Andrews was among the fortunate. In 1869 – the year he published his paper – the Royal Society chose his work as the Bakerian Lecture of that year and thereby brought Andrews and his singular study to prominence. Across the scientific world, important people took notice.
In Scotland, it made a deep impression on James Clerk Maxwell, who was busy writing his textbook Theory of Heat (1871) and devoted several pages to analysing Andrews’ findings.
In the United States, it made a deep impression on Josiah Willard Gibbs, who cited Andrews’ experiments on carbonic acid as supporting evidence for a free energy function in the 1873 paper “A method of geometrical representation of the thermodynamic properties of substances by means of surfaces”.
In the Netherlands it set a physicist thinking. His name was Johannes van der Waals.
And in the rest of Europe, it set off a race among enterprising engineers to liquefy the gases hitherto termed non condensable. As we have seen, that race was won by Louis Cailletet, although in fairness it should be stated that a Swiss physicist called Raoul Pictet also succeeded in liquefying oxygen within days of Cailletet.
The two men used different cooling principles: Pictet opted for enthalpic cooling using liquid SO2 and CO2 while Cailletet employed Joule-Thomson cooling. The advantage of the latter method, as Joule and Thomson had shown during their pioneering experimental work in the 1850s, was that it allowed recirculation of gas cooled by previous passage through the throttle.

Joule and Thomson’s recirculation design from 1853. The red arrow shows the location of the throttle.
This self-intensifying cooling technique was the key to the first large-scale gas liquefaction method developed by William Hampson (1895) and by Carl von Linde (1895), in which the gas was recirculated through a heat exchanger in order to lower the temperature of incoming gas:
In the early days of liquid oxygen production from air, the biggest use by far for the gas was the oxyacetylene torch, invented in France in 1904, which revolutionized metal cutting and welding in the construction of ships, skyscrapers, and other iron and steel structures.

Cylinders of oxygen being loaded on a tractor-trailer truck (1914) owned by the Linde Air Products Company. Courtesy Praxair, Inc.
Another method of commercial liquefaction of air, which employed adiabatic cooling as well as the Joule-Thomson effect, was developed by Georges Claude (1901) in France:
A by-product of the air liquefaction process was neon, which spawned a lucrative new industry in the shape of neon lighting. The first public demonstration of neon lights was at the Paris Motor Show of 1910.
Read Part II
– – – –
Related blog posts
Joule, Thomson, and the birth of big science
The story of how Joule and Thomson’s extraordinary collaboration in the 1850s propelled experimental research into the modern era. The second part of this post also explains the thermodynamics of the Joule-Thomson effect.
Joule, Thomson, and trouble with the neighbours
The story of how Joule and Thomson came to form their historic partnership, their increasingly ambitious research in Manchester, and the unfortunate circumstance that derailed it.
Links to original papers
Thomas Andrews, “On the Continuity of Gaseous and Liquid States of Matter”, Phil. Trans. R. Soc. Lond. 1869;159:545-590
The paper that challenged the prevailing notion of non condensable gases, and opened the way to a new era of cryogenic science. It also led to deeper understanding of the thermodynamics of real gases [to be explored in Part II of this blogpost]
Suggested further reading
“Liquefaction of gases – Cailletet’s Experiments” Scientific American, Vol. XXXVIII No.8, February 23, 1878
A detailed contemporaneous account of Cailletet’s experimental apparatus, method and results.
Andrea Sella, “Pictet’s liquefier”
Raoul Pictet deservedly had joint priority with Louis Cailletet for the first liquefaction of a ‘non condensable’ gas, namely oxygen. Here is his story, as told by Professor Andrea Sella of University College London.
T. O’Conor Sloane, “Liquid Air and the Liquefaction of Gases” (1899)
A wonderful period piece made available online at archive.org by the Omania University in Hyderabad, India. The picture reproduction is awful, but for pure historical interest it’s well worth delving into. Sloane’s writing style is a fascination in itself.
– – – –
P Mander February 2014





Hello Anushka and thank you for the kind comment and question. I hope you found most of the answer in Part II, but perhaps it is worth summarizing these points:
– the work of Andrews (1869) got James Thomson thinking (1871) whether a single pressure volume equation could account for both the continuous and discontinuous isothermal curves. Unifying things is a popular pursuit in physics.
– Johannes van der Waals was in the right place at the right time to take up the challenge in his doctoral thesis (1873). The title of his thesis shows that his approach was based on the unifying assumption of continuity of state; his equation is thus a continuous function.
– van der Waals equation is therefore invalid in regions of discontinuity. The function can still be graphed in these regions but will produce a cubic curve that does not reflect physical reality. Note that in the discontinuous region of the CO2 isotherms, the liquid and vapor phase coexist so there is only one degree of freedom according to the Phase Rule: C + 2 – P = 1 + 2 – 2 = 1. Since the temperature is independently chosen, the pressure will be invariant i.e. a straight line parallel to the x-axis.
LikeLike
This was so useful! but i have a doubt
as in andrew’s isotherms(PVsV plot) during phase transition from gas to liquid it’s a straight line parallel to x axis, but later james thompson thought to replace it with a rather smooth loop which intersects the line parallel to x axis drawn at a given pressure at 3 points. from this vander waal’s formulated equation of state for a real gas having three solutions.
when experimentally the graph is a straight line parallel to x axis during phase transition what’s the need to change it to a loop?
how could we explain the straight line observed experimentally with the vander waal’s equation having only three solutions?
for more clear concept written above go through the link below👇
https://www.jstor.org/stable/3557692?read-now=1&seq=1#page_scan_tab_contents
please answer🙏🙏🙏
LikeLike
Meanwhile in Australia, the beer was already chilled thanks to James Harrison’s patented vapour compression refrigeration, circa mid 1850’s.
LikeLike
It is interesting to notice that two discoveries cited here gave birth later to two “big” compagnies : Linde AG by Carl von Linde himself in 1879 and Air Liquide from the patent of Georges Claude in 1902. Bottom line : these compagnies are nowadays the two main manufacturers of gas liquefying systems, mostly used for natural gas and helium.
LikeLiked by 1 person
Reblogged this on nebusresearch and commented:
I know, or at least I’m fairly confident, there’s a couple readers here who like deeper mathematical subjects. It’s fine to come up with simulated Price is Right games or figure out what grades one needs to pass the course, but those aren’t particularly challenging subjects.
But those are hard to write, so, while I stall, let me point you to CarnotCycle, which has a nice historical article about the problem of liquefaction of gases, a problem that’s not just steeped in thermodynamics but in engineering. If you’re a little familiar with thermodynamics you likely won’t be surprised to see names like William Thomson, James Joule, or Willard Gibbs turn up. I was surprised to see in the additional reading T O’Conor Sloane show up; science fiction fans might vaguely remember that name, as he was the editor of Amazing Stories for most of the 1930s, in between Hugo Gernsback and Raymond Palmer. It’s often a surprising world.
LikeLiked by 1 person