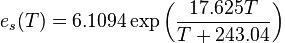The above image from the northern latitudes of Mars, taken by the Viking 2 Lander in May 1979, revealed the presence of a white substance on and around the red rocks. We get this white stuff on Earth too. It’s called frost.
I can remember when I first saw this image confirming the presence of water frost on the Martian surface, I took an instant step closer to believing in the possibility of Little Green Men. During the Martian summer, the temperature can rise above freezing point, and lo! there would be liquid water, the swimming pool of life.
After revelling in this idea for a while, the thought struck me that I had directly transferred the phase change behavior of water on Earth to another planet. On reflection however, this appeared perfectly valid. There are no planet-dependent terms in the Clausius-Clapeyron equation, so the phase diagram of water must look exactly the same up there as it does down here.
Sound enough reasoning. The phase diagram is a PT plane, and according to what I was taught, those thermodynamic variables, along with state functions such as enthalpy of fusion, were considered to apply universally. But then duh! the realization dawned on me – I had failed to consider differences in atmospheric pressure.
At the time, I knew nothing at all about the atmosphere on Mars, apart from the fact that it had one. But at least I could calculate the minimum surface pressure that the atmosphere would need to exert in order for water to undergo a solid-liquid phase change at 0°C. The Magnus-Tetens approximation, which generates saturated vapor pressure (hPa) as a function of temperature (°C)
made things simple since when T=0 the answer is immediately 6.1094 hectopascals, or if you prefer, 6.1094 millibars.
Back then, I never did find out what the atmospheric pressure on Mars was, and my inquiry stalled. Years later I came upon the information by happenstance. The atmospheric surface pressure on Mars is just below 6.1 hectopascals.
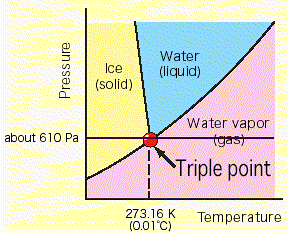
Now a glance at the phase diagram for water shows that the triple point temperature is a gnat’s whisker from 0°C, so for practical purposes the triple point pressure can be taken as 6.1 hPa. This is significant because at no point in the PT plane below the horizontal drawn through the triple point does the liquid phase exist. Since the atmospheric surface pressure on Mars lies below this horizontal, the only possible phase change is between solid and vapor. No liquid water can exist on the surface of Mars.
Except. Well, there are craters on Mars, so if at the bottom of a sufficiently deep one the atmospheric pressure were to nudge above 6.1 hPa, and if the temperature crept above 0°C, and if there was surface frost to start with, then liquid water would form. That’s a lot of ifs, but a possibility all the same. There might not be Little Green Men down there, but there might be something else that answers the description of ‘life form’.
– – – –
Postscript

April 2021: Nearly 8 years after penning this post, I am returning to record the astonishing happenings on Mars with the arrival of the Mars Rover and the amazing helicopter Ingenuity. In similar vein to the blog post above, I immediately thought in earthbound terms when I first saw the little chopper take off. It was only later the thought struck me that I was able to infer from Ingenuity’s motion that Mars has an atmosphere. You can’t see it but the chopper provides visual proof that it is there.
Historial note: Ingenuity flew a total of 72 missions, the first on Monday 19th April 2021 and the last on Thursday 18th January 2024.
– – – –
Photo credits: (top) Mars, Ingenuity / NASA, (below) Little Green Men / Wikimedia
Triple point diagram credit: National Metrology Institute of Japan
(I have added the horizontal line through the triple point)